In mid-April, while multiple vaccine teams around the world announced progress, China announced that the first wave of the epidemic had been brought under control, and the proportion of clinical trials for China ’s new crown research was reduced from two-thirds to half.
Zhong Nanshan, an epidemiologist in China, said, “There is no such opportunity for large-scale clinical drug or treatment research in China.”
Outside of China, WHO has taken the lead in coordinating resources to develop vaccines, and GlaxoSmithKline (GSK), the world’s two largest pharmaceutical manufacturers, and Sanofi, France, have announced joint research and development of vaccines. British scientists have allowed vaccines to be produced on a small scale before clinical results are available.
China missing out on research opportunities
In the WHO updated statistics on April 15, the number of global clinical trials has increased from 927 on April 7 to 1,135. The global proportion of China-related clinical trials has dropped from nearly 2/3 a week ago to nearly 1/2.
Chen Zhengming, a professor of epidemiology at Oxford University in the UK, told BBC Chinese: “The clinical trials in China during the first two months attracted global attention. There was no coordination and wasted resources.”
As of April 16, according to data from the Chinese media website “Observer Network”, 598 clinical trials of new coronavirus have passed the project initiation. At the same time, more than 40 projects were cancelled.
Chen Zhengming said: “There are too many projects at once, and each project needs patients, and in the end it is done piecemeal. One project may use ten, twenty or even one hundred patients, but no problem can be solved.”
On April 15, two clinical trials of Remdsivir, known as “the hope of the people”, were “suspended” in China, because “the current epidemic of new coronary pneumonia has been well controlled, and no eligible patients have entered group”.
Zhong Nanshan, head of the China National Health and Safety Commission’s high-level expert group, pointed out in the “Science News”: “Many studies have been cancelled because no one can think that China will control the epidemic so quickly. Now we need to conduct large-scale clinical trials in China. There is no such opportunity for medicine or treatment research. ”
Zhong Nanshan also admitted: “Now in China, we are very difficult to collaborate, this is my own experience.” He said, (China) a national chess game to collaborate on some of the most important issues, on the surface, but in fact some are No way.
Chen Zhengming believes that there is no coordination in China, and everyone is doing their own thing, and it will be impossible to get things done in the end, resulting in a great waste of resources and opportunities
x ray machine mobile dr is Powerful and easy to operate,More accurate detection!
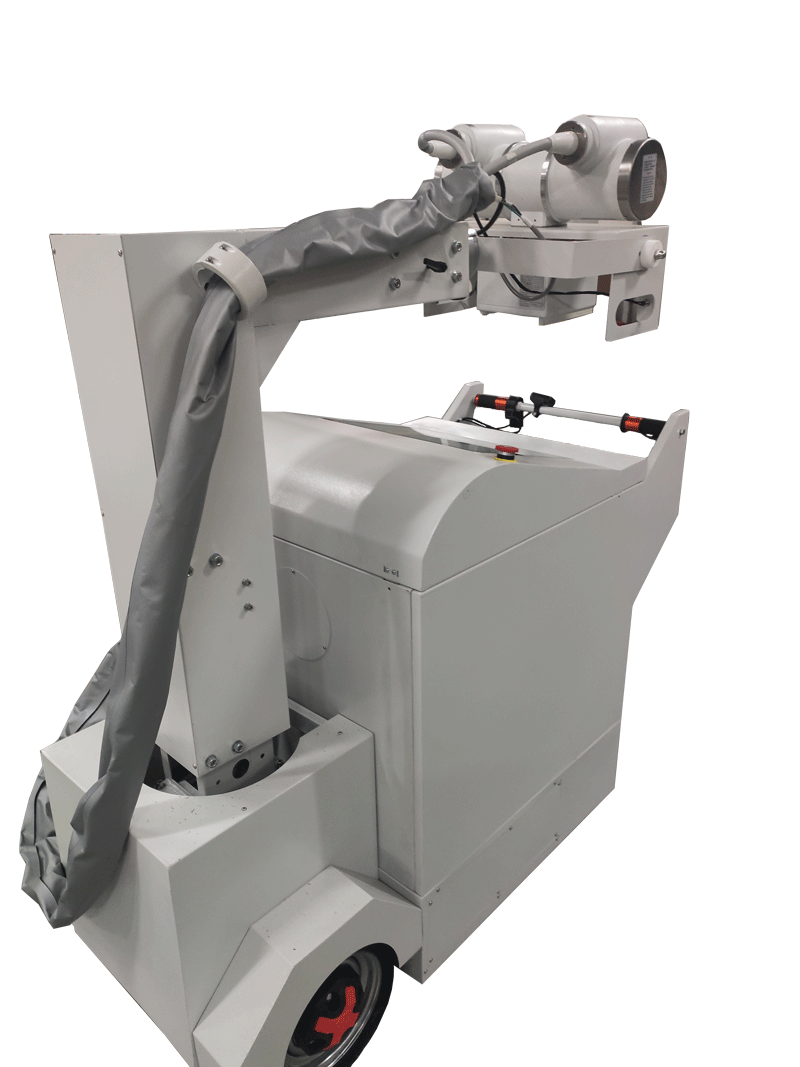
Mobile DR X-ray Machine
Hot Line: +86 536-8882360
Email: service@newheek.com
Company: Weifang Newheek Electronic Tech Co., Ltd.
Add: E Building of Future Star Scientific Innovation Industrial Zone of No.957 Wolong East Street, Yulong Community, Xincheng Sub-District Office, Weifang Hi-tech Zone, Shandong Province, China